Pharmaceutical Cold Chain Management
Maintaining the integrity of the cold chain is crucial in the highly regulated and critical pharmaceutical business. The pharmaceutical cold chain ensures that temperature-sensitive medicines, such as vaccines and biopharmaceuticals, are stored, handled, and delivered within the proper temperature range to retain quality, efficacy, and safety. Any temperature change has the potential to induce product degradation, resulting in major health risks and financial losses.
With an emphasis on precision and reliability, our solutions are precisely developed to monitor and manage the environmental conditions of pharmaceutical products. From manufacture to delivery, our solutions are critical in ensuring that temperature-sensitive items reach end consumers without sacrificing quality.
Products and their Application
Lascar Electronics provides an extensive and innovating range of solutions that have been carefully developed to fulfil the pharmaceutical cold chain’s varying needs:
USB Data Loggers
These loggers successfully ensure that any deviations from the required temperature are recorded and monitored.
-
Temperature Data Loggers
-
Temperature Data Loggers
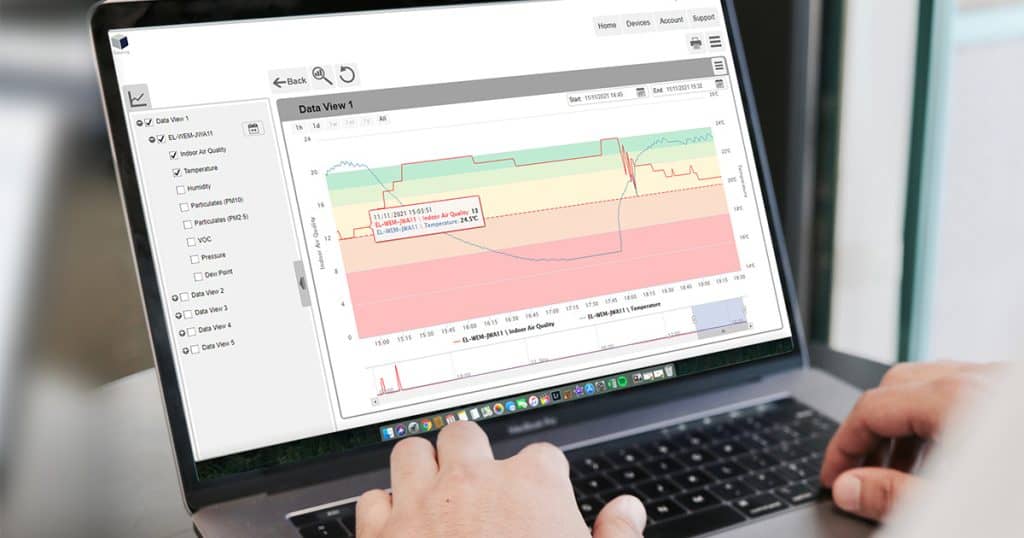
Wireless Data Loggers
These are especially useful for large storage facilities or circumstances requiring remote monitoring. These advanced loggers transmit essential temperature data in real time to a centralized system, allowing for fast and informed corrective actions.
-
Temperature Data Loggers
-
Vaccine Monitoring
-
Vaccine Monitoring
Cold Chain Logistics Loggers
These loggers have been designed to solve the logistical complexities of the pharmaceutical cold chain. They are critical in closely monitoring and maintaining the essential conditions during the difficult transportation journey of pharmaceutical items, ensuring compliance with important temperature restrictions and keeping the products’ inherent quality.
-
Cold Chain Logistics
Cold Chain Logistics, Temperature & Humidity
US$391.99 ex. taxPack of 10 Platelet Temp & RH LoggerAdd to cart -
Cold Chain Logistics
Cold Chain Logistics, Temperature Data Loggers
US$277.99 ex. taxPack of 10 Ambient Pharma Goods Data LoggerAdd to cart
Why Choose Lascar Electronics?
Several key aspects distinguish Lascar Electronics:
Years of Experience: With a rich heritage of technological innovation and an in-depth comprehension of the unique and evolving demands of Pharmaceutical Cold Chain Management.
User-Centric Design: Lascar’s products are carefully developed with the end-user in mind, ensuring seamless ease of use, transparent monitoring and correct data interpretation.
Customizable Solutions: Recognizing the diverse and specific needs inherent in different cold chains, Lascar proactively offers adaptable and tailored solutions, ensuring perfect alignment with individual operational requirements.
Dedicated Support: Lascar Electronics demonstrates a dedication to consistent customer service, responding quickly to requests and fixing any unforeseen concerns, delivering a seamless and engaging user experience.
Case Study
The upcoming case study section will provide an in-depth examination of a specific scenario in which Lascar’s cutting-edge technology played a transformative role.
WiFi: Ashtons Hospital Pharmacy
Monitoring all temperatures from one central point
EL-WiFi Loggers have been proven to be a very useful tool for our business. The fact that we are now able to monitor all of our temperature loggers from one central online platform has been a huge benefit for our operations both in terms of the time efficiency of our temperature monitoring and the efficacy of our compliance measures” – Kevin Fox – Director of Operations, Ashton’s Hospital Pharmacy Services.
Featured Products
-
Vaccine Monitoring
-
Cold Chain Logistics
Cold Chain Logistics, Temperature & Humidity
US$391.99 ex. taxPack of 10 Ambient Pharma Temp & RH LoggerAdd to cart -
Cold Chain Logistics
Cold Chain Logistics, Temperature & Humidity
US$391.99 ex. taxPack of 10 Blood Temp & RH LoggerAdd to cart -
Vaccine Monitoring
-
Cold Chain Logistics
Cold Chain Logistics, Temperature Data Loggers
US$277.99 ex. taxPack of 10 Ambient Pharma Goods Data LoggerAdd to cart -
Vaccine Monitoring